
Molar Mass / Molecular Weight of Sodium carbonate decahydrate YouTube
Solution Verified by Toppr The molar mass of sodium carbonate is 105.987 g / m o l Explanation: To calculate the molar mass of a compound, multiply the subscript of each element from the formula times the molar mass of the element. The molar mass of an element is its atomic weight (relative atomic mass) on the periodic table in g/mol.
Molar Mass Of Sodium / Molar Mass of Na2Co3_Periodic Table StudyGate Blog H2so4 + 2naoh →
The first step to finding the molar mass of Sodium Carbonate is to count the number of each atom present in a single molecule using the chemical formula, Na2CO3: 2. Find Atomic Mass of Each Element Next, using the periodic table, find the atomic mass in g/mol of each element (the molar mass of an element is equal to its atomic mass): 3.

What is the molar mass of sodium bicarbonate, ? YouTube
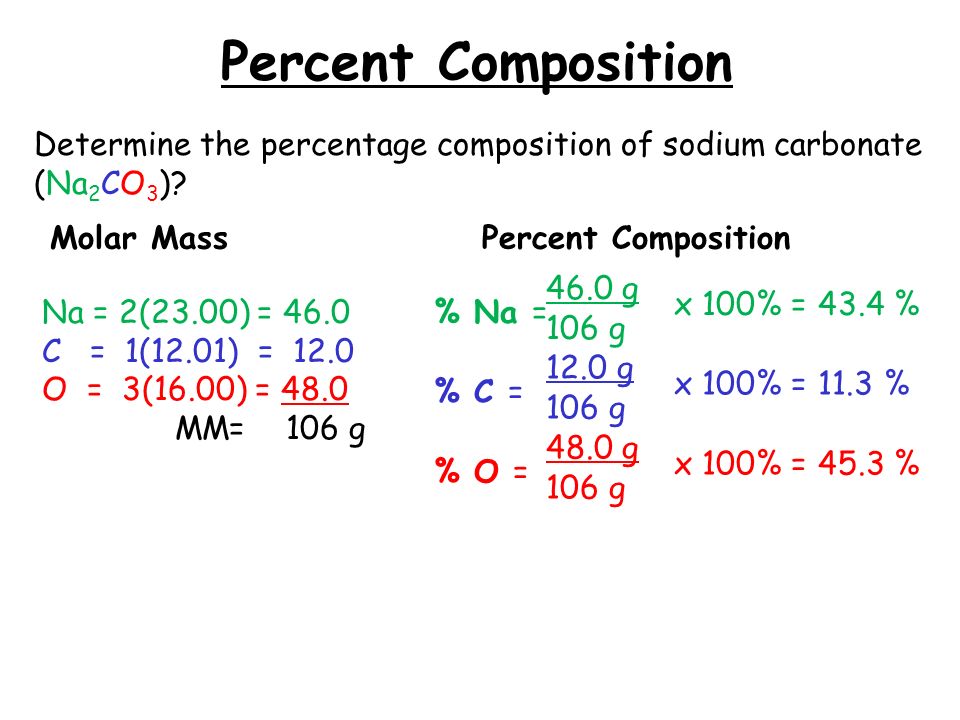
You can see the molar mass value of all the atoms from this periodic table. Now in Na2CO3, there are 2 Sodium atoms, 1 Carbon atom and 3 Oxygen atoms. So let's look at the molar mass of Sodium, Carbon and Oxygen from the above periodic table. You can see that; The molar mass of Sodium is 22.990 g/mol. [1]

How to find the molar mass of Na2CO3 (Sodium Carbonate) YouTube
2005-06-24. Modify: 2024-01-06. Description. Sodium carbonate decahydrate is an organooxygen compound. ChEBI. Natrite is a mineral with formula of Na 2 CO 3 or Na 2 (CO 3 ). The corresponding IMA (International Mineralogical Association) number is IMA1981-005. The IMA symbol is Nat.

Answered 1. Calculate the molar mass of sodium… bartleby
Molar mass: 105.9888 g/mol (anhydrous) 286.1416 g/mol (decahydrate) Appearance White solid Odor: Odorless Density: 2.54 g/cm 3 (25 °C, anhydrous) 1.92 g/cm 3 (856 °C). Sodium carbonate, sometimes called washing soda or soda ash, is an inorganic weak base with chemical formula Na 2 CO 3.

PPT Chemistry Unit 7 The Mole PowerPoint Presentation, free download ID6231872
The mass of 1 mole of a substance is called its molar mass. Step2. Formula for molar mass. Molar mass = The atomic mass of element × number of atoms given in subscript. Step3. Molar mass of Sodium carbonate Na 2 CO 3. Molar mass of Na 2 CO 3 = 2 × The atomic mass of Na+ The atomic mass of C+ 3× The atomic mass of O. Hence, the molar mass of.

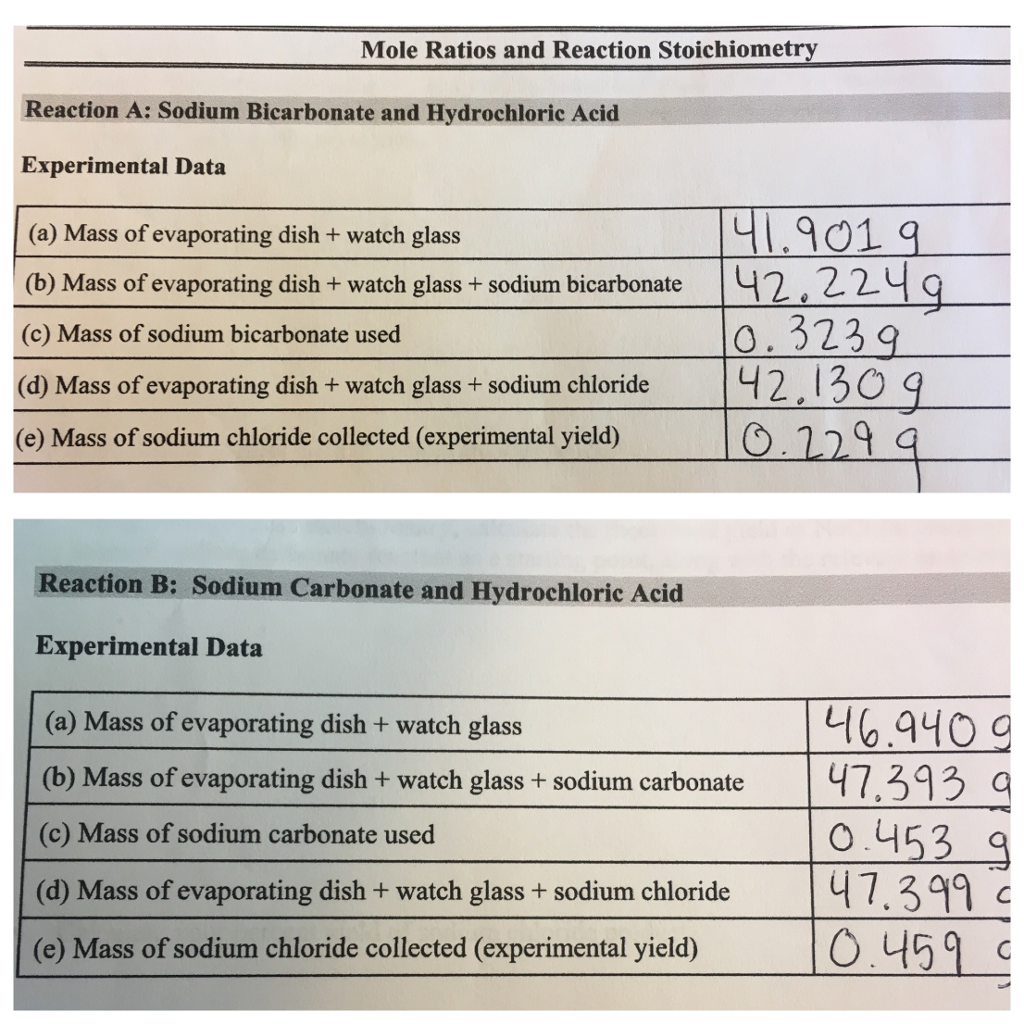
Calculate the mass of sodium carbonate to prepare (molecular mass of sodium carbonate=106g and
Explanation of how to find the molar mass of Na2CO3 • 10H2O: Sodium carbonate decahydrate.A few things to consider when finding the molar mass for Na2CO3 • 1.

Calculate molecular mass Na2CO3.10H2Omolar mass Na2CO3Sodium carbonate Molar mass YouTube
Element: Sodium Symbol: Na Atomic Mass: 22.989770 # of Atoms: 2 Mass Percent: 43.382% Element: Carbon Symbol: C Atomic Mass: 12.0107 # of Atoms: 1 Mass Percent: 11.332% Element: Oxygen Symbol: O Atomic Mass: 15.9994 # of Atoms: 3 Mass Percent: 45.286% Similar chemical formulas Note that all formulas are case-sensitive.

Sodium Carbonate Molar Mass ChaimhasVance
Sodium Carbonate Molar Mass. To determine the molar mass of sodium carbonate (Na2CO3), the individual atomic masses of all its constituent elements are determined. Sodium (Na) is 22.99 g/mol. Carbon (C) is 12.01 g/mol. Oxygen (O) is 16.00 g/mol.

Molar Mass / Molecular Weight of NaHCO3 Sodium hydrogen carbonate YouTube
Soda Ash Disodium carbonate Carbonic acid disodium salt View More. Molecular Weight 105.988 g/mol Computed by PubChem 2.2 (PubChem release 2021.10.14) Parent Compound CID 767 (Carbonic Acid) Component Compounds CID 5360545 (Sodium) CID 767 (Carbonic Acid) Dates Create: 2005-07-19 Modify: 2023-12-30 Description
How Do You Find The Molar Mass Of Sodium Bicarbonate
xplanation of how to find the molar mass of NaHCO3: Sodium bicarbonate (sodium hydrogen carbonate).A few things to consider when finding the molar mass for N.

Sodium Carbonate Molar Mass, Formula, and Uses
Sodium carbonate is a diazonium salt of carbonic acid with the chemical formula Na2CO3. It is also known as Soda crystals, soda ash, washing soda. This inorganic compound is water-soluble and when dissolved in water, it forms carbonic acid and sodium hydroxide. In its pure form, it is a white powder and odourless.

What is the mass of 5 moles of sodium carbonate ( Na2co3) Brainly.in
47.997. Total Mass. 105.986. The molar mass of Na2CO3 (Sodium carbonate) is: 105.986 grams/mol. See also our theoretical yield calculator for chemical reactions (probably your next stop to finish the problem set).

Solved 7. What Is The Molar Concentration Molar Mass/g.mo...
Sodium carbonate (also known as washing soda, soda ash and soda crystals) is the inorganic compound with the formula Na2CO3 and its various hydrates. All forms are white, odourless, water-soluble salts that yield alkaline solutions in water.

Changes in molar mass distribution of sodium carbonate soluble pectin... Download Scientific
Molecular mass ( molecular weight) is the mass of one molecule of a substance and is expressed in the unified atomic mass units (u). (1 u is equal to 1/12 the mass of one atom of carbon-12) Molar mass ( molar weight) is the mass of one mole of a substance and is expressed in g/mol.

PPT Stoichiometry PowerPoint Presentation, free download ID3919556
Explanation of how to find the molar mass of Na2CO3: Sodium carbonate.A few things to consider when finding the molar mass for Na2CO3:- make sure you have th.